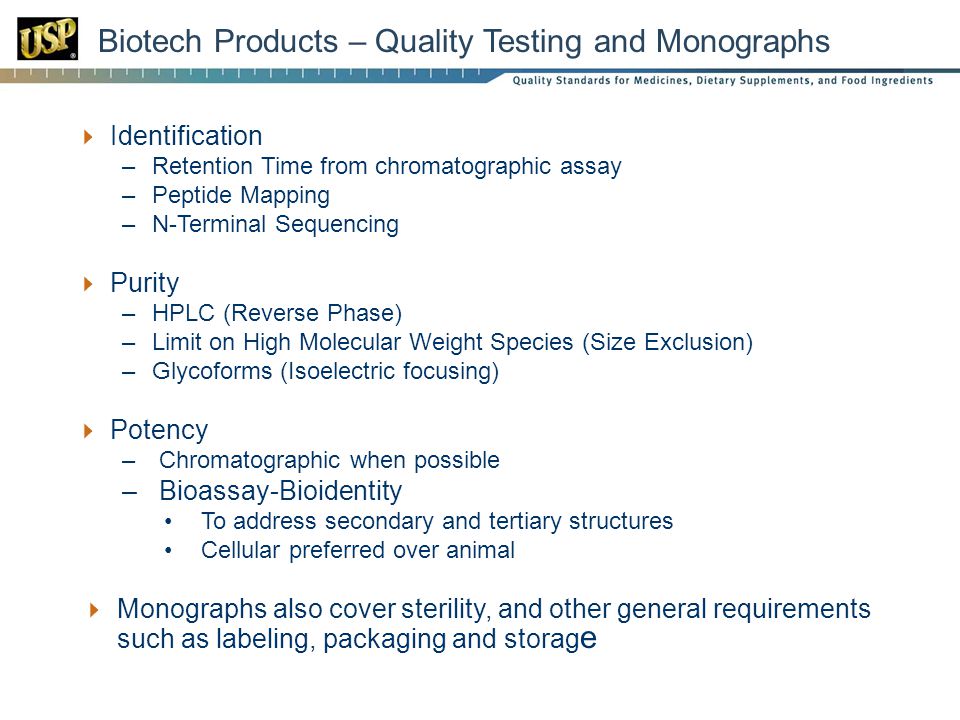
Usp Monograph Filgrastim
Jul 11, 2013 - filgrastim for Reducing the Duration of Severe Neutropenia. By the US Pharmacopeia (USP) definition, a biologic is produced in a living. USP Product-Class Standards for Biologics. The flexible monograph mechanism allows the accommodation of multisource materials as well as the application of different, yet equivalent, procedural approaches to the determination of established quality attributes for a given molecule. USP has been developing, and continues to develop.
Pnas article word template. In this report, we emphasize the importance of public monographs with reference materials, coupled with careful process and change control and attention to GMPs, as a means of advancing access to good quality, safe, and effective medicines, with emphasis on available and incoming biologic medicines. With adequate control of articles covered by a monograph, these public standards can form the basis for a global public quality platform that covers reference products, non-interchangeable reference products, biosimilars, and interchangeable biosimilars. Working collaboratively with all stakeholders, new approaches allow these public standards to emerge nationally and globally in a timely way. Yet, there are increasing limitations in the availability of public standards for biologic medicines, which may reverse many decades of progress. Solutions are considered in this report. INTRODUCTION Biologic medicines are an increasing component of the therapeutic armamentarium of practitioners, with a long and distinguished history of value to patients/consumers. The increase in the medicinal value of biologics is expected to expand with improved understanding of human and animal biology and rising capability of manufacturers to make safe, effective, and good quality biologics through recombinant technology.
Equally important, global, regional, and national approaches to first-entry reference products, other reference products with the same active moiety but without comparison to the first (referred in this article as a non-interchangeable reference products), biosimilars, and interchangeable biosimilars, with the understanding that a global ingredient and product quality platform can be developed that is suitable for all four categories (Fig. ). We argue that a key component of this platform is a public monograph with allied reference materials that, for potency, are traceable to a World Health Organization (WHO) biological reference material (BRM), denoted as a WHO international standard or reference reagent ().
This report follows a larger paper published by the US Pharmacopeial Convention (USP) that considered primary and secondary standards, together with monographs, for food and drug articles (). Four categories are emerging globally for a biologic product: (1) reference product, (2) one or more non-interchangeable reference products, (3) one or more biosimilar products to each reference product, and (4) interchangeable biosimilar reference products (USA only). All can be linked via common unitage traceable to an available WHO biological reference material with specified unitage. For categories 1 and 2, clinical trial designs might be based either on demonstrating a difference (two-sided) or non-inferiority (one-sided). For categories 3 and 4, designs are based on demonstrating similarity with criteria for population or individual equivalence.
The dark shaded area in the figure denotes varying degrees of intellectual property protection via either patent or exclusivity, ranging from none to a minimum of 12 years exclusivity in the USA via BPCIA. LIMITATIONS ON AVAILABILITY OF BIOLOGIC PUBLIC STANDARDS Historically, WHO provides both written and measurement standards for biological medicines, e.g., vaccines and therapeutic proteins. To perform this function, WHO works with various collaborating centers, including the UK’s National Institute for Biologic Standardization and Control (NIBSC, which recently joined the UK’s Medicines and Health Care Products Regulatory Agency) to prepare BRMs that maintain the unitage of both natural source and rDNA biologic medicines (). These BRMs are critically important to global and national health as a means of assuring consistency in potency over many years of manufacture and during shelf life. The WHO BRM represents a global primary standard to which a national pharmacopeia or similar standard becomes secondary. Tertiary standards may be developed as working standards by pharmaceutical manufacturers. An excellent review notes the importance of unitage for insulin, an archetypal biologic ().
Typically, the potency test forms the core of a public monograph together with additional physicochemical tests. This approach has worked well, with many BRMs available from WHO (). Advancing BRMs is becoming more challenging for the newest biological medicines, such as monoclonal antibodies, fusion proteins, and other highly engineered biologics, as there generally is no readily available donor other than a first-entry manufacturer. These molecules, some of which are bioengineered versions of earlier molecules, do not exist in nature, unlike the case for earlier proteins such as insulin, somatotropin, and erythropoietin. However, with the development of multi-source products for the same active moiety, there is an urgent need for standards for monoclonal antibodies and bioengineered molecules ().
Free Download 12 Rounds 2009 Tamil Dubbed Movie Download TamilRockers,12 Rounds 2009 Tamil Dubbed Movie Download TamilRockers Download, 12 Rounds (2009) Tamil Dubbed English BDRip TamilRockers,12 Rounds 2009 Tamil Dubbed Movie Download TamilRockers TamilRockers 480p HD Bluray, Tamil Dubbed Movie Download, playtamil, tamilplay. Hitcric. 12 Rounds (2009)[720p - BDRip - [Tamil + Eng] - x264 - 900MB - ESubs] click on the image to view full size.